Radioactive decay, also known as nuclear decay or radioactivity, is the process by which a nucleus of an unstable atom loses energy in the form of radiations. A material that spontaneously emits this kind of radiation - which includes the emission of energetic alpha particles, beta particles, and gamma rays - is considered radioactive. When unstable nuclei decompose in nature, the process is referred to as natural radioactivity or spontaneous radioactivity. When the unstable nuclei are prepared in the laboratory, the decomposition is called induced radioactivity.
Fission is a splitting of something into two parts.
In nuclear physics and nuclear chemistry, nuclear fission is either a nuclear reaction or a radioactive decay process in which the nucleus of an atom splits into smaller parts (lighter nuclei).
The fission process often produces free neutrons and photons (in the form of gamma rays), and releasing a very large amount of energy. When a nucleus fissions, it splits into several smaller fragments. These fragments, or fission products, are about equal to half the original mass. Two or three neutrons are also emitted. The sum of the masses of these fragments is less than the original mass. This 'missing' mass has been converted into energy according to Einstein's equation:
E = mc2
The Fission Process
A neutron travels at high speed towards a uranium-235 nucleus. A neutron travels towards a uranium-235 nucleus. The neutron strikes the nucleus which then captures the neutron. The nucleus changes from being uranium-235 to uranium-236 as it has captured a neutron. The uranium-236 nucleus formed is very unstable. It transforms into an elongated shape for a short time.
The uranium-236 nucleus formed is very unstable. It transforms into an elongated shape for a short time. The uranium-236 nucleus formed is very unstable. It transforms into an elongated shape for a short time. It then splits into 2 fission fragments and releases neutrons. It then splits into 2 fission fragments and releases neutrons.
Nuclear Chain Reactions:
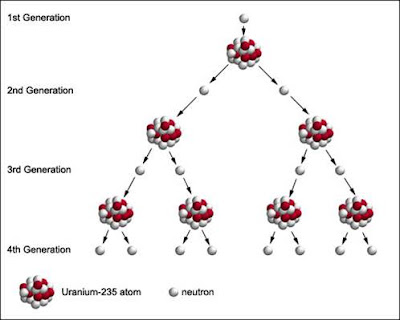
A chain reaction refers to a process in which neutrons released in fission produce an additional fission in at least one further nucleus. This nucleus, in turn, produces neutrons, and the process repeats. The process may be controlled (nuclear power) or uncontrolled (nuclear weapons).
Critical mass:
 |
| Image from University of Florida |
U235 + n → fission + 2 or 3 n + 200 MeV
If each neutron releases two more neutrons, then the number of fissions doubles each generation. In that case, in 10 generations there are 1,024 fissions and in 80 generations about 6 x 10 23 fissions.
1 MeV (mega electron volts) = 1.609 x 10 -13 joules
Uranium-235 combines with a neutron to form an unstable Uranium-236, which quickly splits into barium-144 and krypton-89 plus three neutrons in the process of nuclear fission.
 |
| Image from University of Dehli |
Energy from Fission
Both the fission fragments and neutrons travel at high speed. The kinetic energy of the products of fission are far greater than that of the bombarding neutron and target atom.
EK before fission << EK after fission
Energy is being released as a result of the fission reaction. The energy released can be calculated using the equation:
E = mc2
Where:
E = energy released (J)
m = mass difference (kg)
c = speed of light in a vacuum (3 x 108 ms-1)
The energy released from this fission reaction does not seem a lot. This is because it is produced from the fission of a single nucleus. Large amounts of energy are released when a large number of nuclei undergo fission reactions. Each uranium-235 atom has a mass of 3.9014 x 10-25 kg.
The total number of atoms in 1 kg of uranium-235 can be found as follows: No. of atoms in 1 kg of uranium-235 = 1/3.9014 x 10-25. No. of atoms in 1 kg of uranium-235 = 2.56 x 1024 atoms
If one uranium-235 atom undergoes a fission reaction and releases 2.385 x 10-11 J of energy, then the amount of energy released by 1 kg of uranium-235 can be calculated as follows:
total energy = energy per fission x number of atoms
total energy = 2.385 x 10-11 x 2.56 x 1024
total energy = 6.1056 x 1013 J
Critical mass:
Although two to three neutrons are produced for every fission, not all of these neutrons are available for continuing the fission reaction. If the conditions are such that the neutrons are lost at a faster rate than they are formed by fission, the chain reaction will not be self-sustaining.
At the point where the chain reaction can become self-sustaining, this is referred to as critical mass.
In an atomic bomb, a mass of fissile material greater than the critical mass must be assembled instantaneously and held together for about a millionth of a second to permit the chain reaction to propagate before the bomb explodes. To maintain a sustained controlled nuclear reaction, for every 2 or 3 neutrons released, only one must be allowed to strike another uranium nucleus.
If this ratio is less than one then the reaction will die out; if it is greater than one it will grow uncontrolled (an atomic explosion). A neutron absorbing element must be present to control the amount of free neutrons in the reaction space.
Most reactors are controlled by means of control rods that are made of a strongly neutron-absorbent material such as boron or cadmium.
The nuclear force (or nucleon–nucleon interaction or residual strong force) is the force between two or more nucleons. It is responsible for binding of protons and neutrons into atomic nuclei. The energy released by such binding causes the masses of nuclei to be less than the total mass of the protons and neutrons which form them; this is the energy used in nuclear power and nuclear weapons. The force is powerfully attractive between nucleons at distances of about 1 femtometer (fm) between their centers, but rapidly decreases to insignificance at distances beyond about 2.5 fm. At very short distances less than 0.7 fm, it becomes repulsive, and is responsible for the physical size of nuclei, since the nucleons can come no closer than the force allows. So, if you feel anything important missed from here so you can comment below and mention How Electrical.





















0 comments:
Post a Comment